Research Interests - Physical Organic ChemistryRecent work in my group has largely been directed towards the effects of host-guest binding on organic reactivity. We are particularly interested in Transition State Stabilization arising from the binding of transition states to "catalysts", such as dendrimers, protons, metal ions, protons, micelles, cyclodextrins and other molecular hosts. In effect, our research is concerned with the origins of Supramolecular Catalysis. |
|
|||
|
|||
|
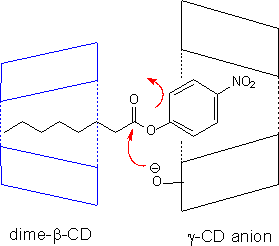
As a result of host-guest complexation, cyclodextrins can alter the reactivity of included molecules in a variety of ways. For examples, consult the reviews: Carbohydr. Res., 1989, 192, 181; Adv.Phys. Org. Chem., 1994, 29, 1; Chem. Rev., 1998, 98, 1997; Chem. Rev., 1998, 98, 2013. Depending on the structure of the reactants and the reaction, complexation of one (or more) of the reactants by a cyclodextrin (CD) may lead to rate acceleration, no effect, or rate retardation. Which of these effects occurs depends on whether the transition state of the reaction is bound more strongly, the same, or less strongly than the initial state (See: Adv. Phys. Org. Chem., 1994, 29, 1).
|
|||
|
|||
|
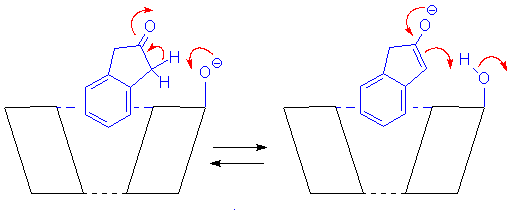
Cyclodextrins catalyze the deprotonation of 2-indanone in basic aqueous solution, presumably through reaction of the ketone with their CD anions (see Figure). The extent of the catalysis depends on the size of the CD and how the ketone sits in its cavity. (OST and R. Donga, J. Chem. Soc. Perkin Trans. 2, 1996, 2763.)
Likewise, the anions of α-CD and β-CD catalyze the deprotonation of β-keto esters, RCOCH2COOR'. For the derivatives studied, binding of the alkoxyl group (O-R') appears to be of more significance than that of the acyl group (R-CO) both in the initial state and in the transition state (OST, N.R. Iyengar and B.K.Takasaki, Can. J. Chem., 1993, 71, 2139). |
|||
|
|||
|
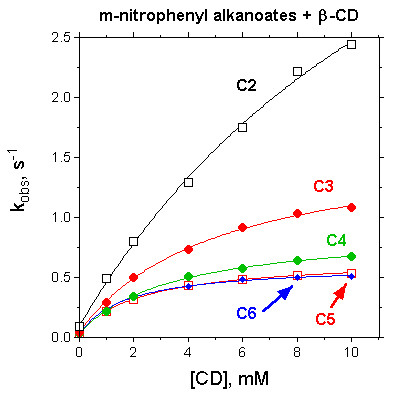
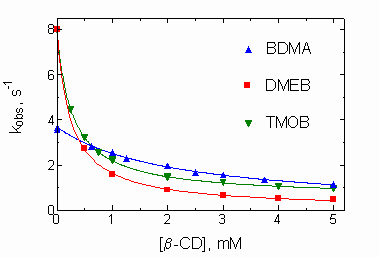
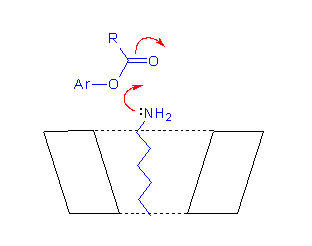
Studies of the effects of cyclodextrins on reactions of non-binding nucleophiles with p-nitrophenyl alkanoate esters (OST & T.A. Gadosy, J.C.S. Perkin Trans. 2, 1994, 2307-2311; J.C.S. Perkin Trans. 2, 1995, 71-76) established that the CD-bound esters have similar or slightly reduced reactivities, compared to the free esters. With alkanoate esters which bind to CDs through their acyl chains, the magnitude of the effects depend on the ester chain length. A later study of the effect of β-cyclodextrin on the reaction of α-amino acid anions with p-nitrophenyl acetate (and the hexanoate) showed modest catalysis of the reactions of those nucleophiles that bind appreciably to the CD (OST, T.A. Gadosy and J.B. Giorgi, Can. J. Chem., 1997, 75, 83-91).
|
|||
|
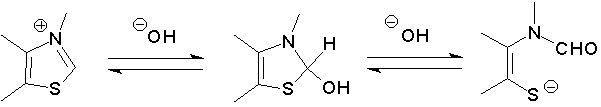
In other, quite different work, done in collaboration of Professor Bob McDonald, of Mount St. Vincent University (Halifax), we have carried out detailed studies of the mechanisms of reversible ring-opening of thiazolium cations in aqueous solution:
Included in this research, which has been published in J. Chem. Soc. Perkin Trans. 2, 1997, 2609-2619, were studies of the ring-opening of Vitamin B1(Thiamine). Professor Kathy Darvesh, also of Mount St. Vincent U., and coworkers have carried out theoretical (ab initio) studies of this type of ring-opening. See article # 13 (OT-313C) in Arkivoc, 2001 (xii). |
|||
|
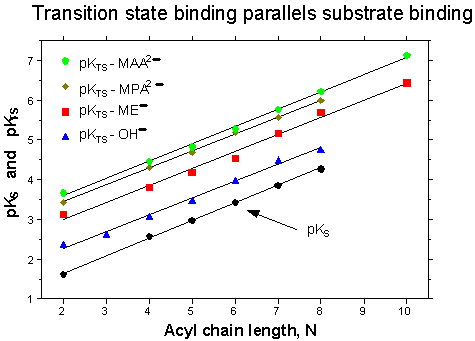
We are also interested in reactions catalyzed or mediated by micelles. Thiolysis of p-nitrophenyl alkanoate esters by the anions of mercaptoethanol (ME), mercaptoacetic acid (MAA), 3-mercaptopropionic acid (MPA), and cysteine (CYST), as well as cleavage by the anion of trifluoroethanol (TFE), are all strongly catalyzed by CTAB micelles in aqueous solution - much more so than is ester cleavage by hydroxide ion or the anion of glycine. The kinetics of ester cleavage are analyzed to give rate constants for nucleophilic anion attack on the esters in the aqueous phase (kN) and the micellar phase (kcN), and dissociation constants (KS) for binding of the esters to the micelles. From these constants we have deduced constants for dissociation of the cleavage transition states bound to the micelles, KTS = kNKS/kcN (cf. Adv. Phys. Org. Chem., 1994, 29, 1).
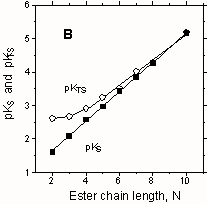
Transition state binding (pKTS) and substrate binding (pKS) are strongly correlated with each other with a slope of ONE because they show the same sensitivity to ester chain length (see Figure above). There is a constant difference between pKTS and pKS them for each nucleophile and so the catalytic ratios (kcN/kN = KS/KTS) are independent of the ester chain. Similar behaviour was found earlier for ester cleavage by hydroxide ion in CTAB micelles (OST & A.A. Fedortchenko, Can. J. Chem., 1997, 75, 1434-1438). It is argued that the ratios kcN/kN vary appreciably with the nucleophiles because of their different propensities to exchange between the aqueous medium and the Stern layer of the CTAB micelles (OST & O.J. Yazbeck, Can. J. Chem., 2000, 78, 1100-1108). |
|||
|
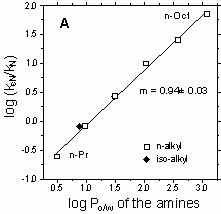
Recently, we have studied the effects of CTAB micelles on ester aminolysis: specifically the cleavage by p-nitrophenyl (pNP) esters by alkyl- amines in water with a constant "ionic atmosphere". The "efficiency" of CTAB-mediated aminolysis of pNP acetate increases systematically with the amine chain length, from a 4-fold retardation (for n-PrNH2) to 70-fold catalysis (for n-OctNH2), and it correlates strongly with amine hydrophobicity, expressed by octanol-water partition coefficients (Fig. A). Also, the strength of transition state binding increases with amine chain length, and it is larger for the pNP hexanoate than for the acetate, suggesting additional (hydrophobic) binding for the hexanoate ester. For the ester series: pNP acetate to decanoate, reacting with n-hexylamine, the catalytic "efficiency" decreases with ester chain length, indicating that the effects of the ester and amine chains are not independent. This decrease in catalysis arises because the chain length dependences of transition state binding and substrate binding are not parallel (Fig. B), unlike the situation with hydrolysis and thiolysis (see Figure above).
Normally, the "concentration effect" arising from partitioning of the reactants into the micelles is sufficient to explain micellar catalysis, but here. In the present case, the rate of amine attack in the Stern layer of CTAB is moderated by strong hydrophobic interactions of the amine and ester with the micellar core when both have long alkyl chains. |
|||
| Acknowledgements:
I am extremely grateful to all my coworkers and
collaborators whose names appear in the abstracts, list of
publications, or on the group webpage. Without their efforts, most of
the research could not have been carried out. Our work has been made possible by operating grants, equipment grants, and scholarships from the Natural Sciences and Engineering Research Council of Canada (NSERC) and by the support of Concordia University. We are also grateful to Wacker Biochem (Wacker-Chemie) for the gift of some Cyclodextrins. © Oswald S. Tee, 1998-2007 E-mail: ostee@alcor.concordia.ca |
|||
 |
Last revised: December 30, 2014 (ost) |