Pawelek Lab
Concordia University, Montreal
Pawelek Lab
Concordia University, Montreal
Research Summary
We study the molecular mechanisms of siderophore-mediated iron uptake. Siderophores are small-molecule iron chelators synthesized and secreted by bacteria under conditions of iron stress to scavenge iron for cell survival. To what extent do the proteins involved in siderophore biosynthesis and secretion engage in protein-protein interactions? Can the disruption of key protein interaction interfaces eliminate bacterial cell growth under iron-limiting conditions? We hope that our research will ultimately lead to the identification of novel targets for the development of a powerful new class of antimicrobial agents to which bacteria do not have resistance. To pursue our research goals, we employ a wide range of techniques including biophysical methods (fluorescence spectroscopy, circular dichroism, AUC, ITC), X-ray crystallography, and in vivo approaches (microbiological assays, intracellular expression of fusions with fluorescent proteins).
Click here for recent publications.

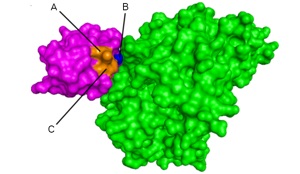
AUTODOCK simulation of a protein-protein interaction formed during enterobactin biosynthesis.
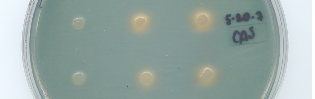
CAS assay showing reduced enterobactin secretion in E. coli knockout strains deficient in enterobactin biosynthesis (left) compared to knockout strains transformed with complementing genes (middle), and to wild-type E. coli (right).
Peter D. Pawelek, Ph.D.
Associate Professor
Department of Chemistry and Biochemistry
7141 Sherbrooke St. West, H4B 1R6
Office: Rm. SP275.33. Tel: 514-848-2424 x3118
Lab: Rm. SP280.27. Tel: 514-848-2424 x5836
Fax: 514-848-2868
Email: peter.pawelek@concordia.ca
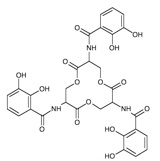
Above: Chemical structure of the siderophore enterobactin.
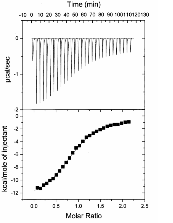
Right: ITC experiment show-
ing binding the enterobactin intermediate DHB to E. coli EntE