 These pages are under construction, again.
These pages are under construction, again.
 These pages are under construction, again.
These pages are under construction, again.
Molecular structure studies
Can a stabilizing, weak bonding interaction in a molecule be the
cause for reactions to occur out of more unfavourable configurations or conformations?
Weak bonding interactions, e.g. hydrogen bonds, are of fundamental importance in chemistry:
in protein folding, in the formation of organic complexes, and in packing motifs of organic
molecules in a crystal, to give but a few examples. They can also be important in the
various stages of a chemical reaction, such as in the formation of a pre-reaction complex
or the stabilization of a transition state. If these interactions occur intramolecularly,
they can be regarded as the simplest form of molecular recognition, a field of continuing
importance, yet there are very few data available on whether they influence reactivity.
The consequences of weak interactions can be shown using
various experimental techniques, but their exact nature can only
be studied through the system's electron density, which allows for the complete
characterization of all bonding interactions. A study of all bonding interactions is a
study of the molecular structure! These kinds of studies are only slowly becoming more common in the
available literature because they are carried out by a comparatively small group of scientists.
Summary of selected studies
- Hydrogen bonding network in dimers of N-sulfinylhydrazines:
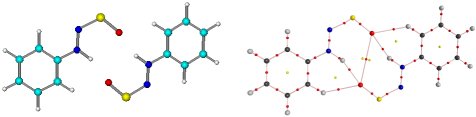
|
Geometry and structure of the Ph-NH-N=S=O dimer. Apart from two N-H...O, two C-H...O interactions are present. Their importance: Dimerization is prevented upon ortho-substitution. P. Malla, MSc 2005 |
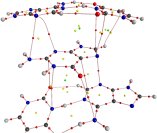 |
Guanine in the binding site of 1Y27. The overlap of the G ligand with bases in floor and ceiling of the aptamer pocket is minimal. Yet, stabilizing p-stacking interactions are revealed. P. Kamya, PhD 2009 |
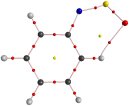 |
Ph-N=S=O in its preferred syn configuration. C-H...O is shown through the electron density... ... and confirmed through NMR temperature studies, where 17O is deshielded and 1H is shielded upon its loss. S. Li, PhD 2009 |
| Return to |
H.M. Muchall HomePage Centre for Research in Molecular Modeling - CERMM |
Last update: august10; hmm