 These pages are under construction, again.
These pages are under construction, again.
 These pages are under construction, again.
These pages are under construction, again.
General
We are interested in electronic and molecular structures of organic molecules, where "molecular" means the
distribution of bonding interactions, with focus on weak interactions such as hydrogen bonds, and
"electronic" means the distribution of electrons as relevant, for example, in resonance structures.
As typical physical organic chemists, we seek an understanding of certain observations and then continue with
"would it not be cool to...". And so general aims of our studies are to
- understand why certain geometries (conformations or configurations) are preferred over others:
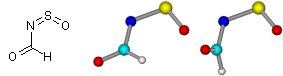
|
A pair of conformational isomers. Which isomer is thermodynamically more stable? Why? |
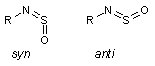 |
A pair of configurational isomers. In all known cases, the anti configuration is much too unstable to be observed. Would it not be cool to change the electronic structure so that anti becomes at least as stable as syn? |
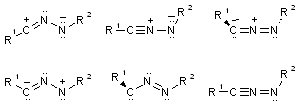 |
Six resonance structures for the connectivity R-CNN-R. Which would be relevant for the parent H-CNN-H? What substitution does it take to get a large carbene component? |
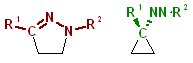 |
R-CNN-R react with alkenes. Reactions are known to be 1,3-dipolar cycloadditions. Would it not be cool to change the electronic structure so that carbene cycloadditions become possible? |
| Return to |
H.M. Muchall HomePage Centre for Research in Molecular Modeling - CERMM |
Last update: august10; hmm